Providers who are required to participate in the quality improvement (QI) program are notified of their QI status when they receive their annual opioid prescribing report in the spring of each year.
Minnesota Department of Human Services (DHS) QI framework follows a simplified version of the improvement model found in the ICSI Opioid Prescribing Quality Improvement Guide.
QI participants are encouraged to contact DHS at any time with questions about their data or the project. Consultations with DHS program staff and contracted medical experts are available by sending an email to dhs.opioid@state.mn.us.
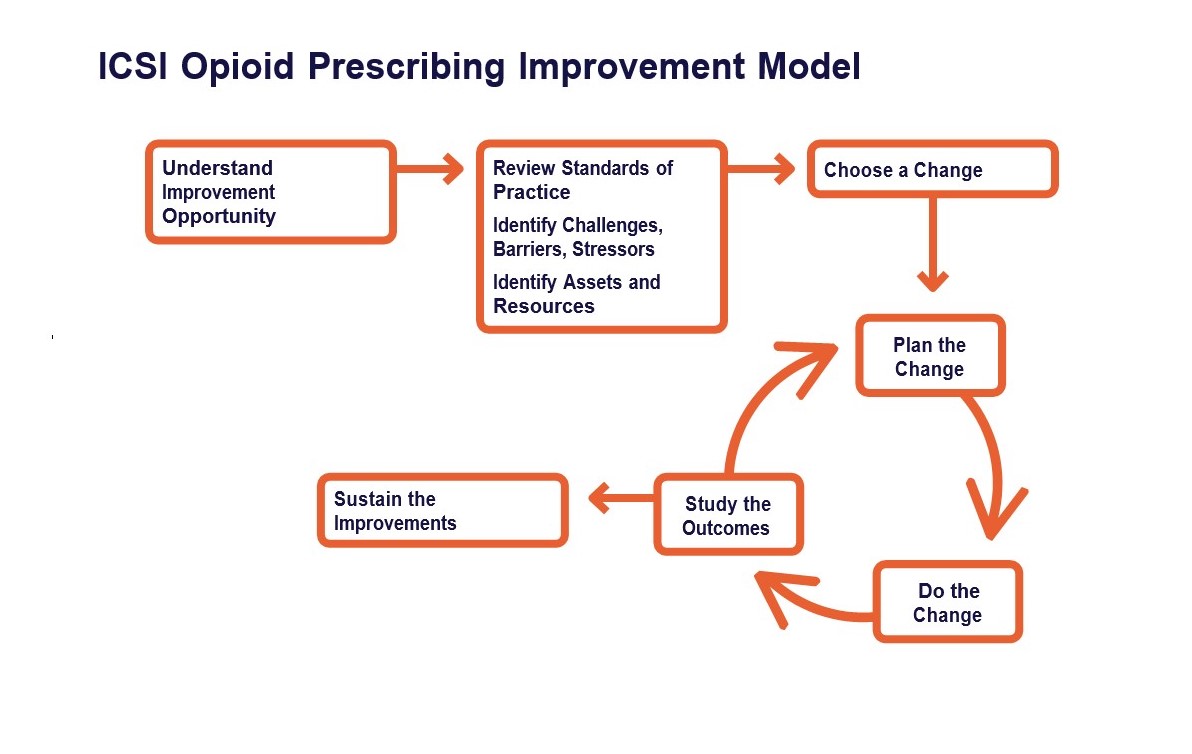
Step 1: Understand the improvement opportunity
Step 2: Review standards of practice; identify challenges, barriers, stressors; choose a change
Step 3: Plan the change
Step 4: Do the change; study the outcomes; sustain the improvements
Chronic pain is a clinically complex phenomenon and individualized, patient-centered care is imperative, whether opioids are part of the treatment plan or not. DHS partnered with a panel of pain management experts to develop a tenable alternative to quality improvement requirements for those who treat chronic pain. The alternative, referred to as a waiver, emphasizes processes that uphold patient safety and clinical practice guidelines.
The waiver is available to clinics specializing in pain management and includes all licensed prescribers on staff, regardless of their engagement in OPIP. Any clinic that meets all the following criteria can apply for a waiver:
Email dhs.opioid@state.mn.us to request an application to apply for a waiver. DHS clinical contractors will review the materials after receiving the waiver application and then make a recommendation for approval or denial to the Assistance Commissioner of the Health Care Administration.